Week 1 - Day 1
How to Pass CH101: the High School Version
- Pay attention, ask questions, complete your homework
How to pass the college version
- Do not take it in the summer
- Read ahead
- You’ll read more new words than when taking a foreign language
- There is more material than anyone can memorize: you need to understand
- Seriously compounding: each new topic builds on previous ones
- DO NOT GET BEHIND
- Go back over each days class
- Reread text book to fix things that were unclear in class
- It’s up to you to learn the material
- Correct / amplify notes as needed
- Join a study group
- Do online homework “as we go”
- Assigned homework is minimum set
- 10-12 hours a week out of class
- Use the practice problems to test yourself
- If you can’t work the practice problems with the textbook closed, then you won’t necessarily do well on the test
- Attempt the problems you can’t immediately see how to work
- Do much more than the minimum
My Labs Plus
- This is where you do your homework and pre-labs
- DO NOT USE THE CODE FOR THE LAB
- You’ll be given that at your lab time
- University of Alabama Login URL: http://ua.mylabsplus.com
- Students Username : MyBama ID (all lower case)
- Students Password : CWID
- Click on your course under the Fall 2016 tab
- Accept Terms of Agreement
- Input Access code (purchased from the bookstore), purchase Access Code, or choose Pay Later to receive 14 days of temporary access
- Your access code is good for 24 months
Syllabus overview
- How fast we cover a section depends on how well we perform to some degree
- Tests are not commutative in the sense that future tests will not ask the same exact questions
- However knowledge of the previous test might be required to answer questions on future tests
- Office hours are Mondays 5-6, Thursdays 4-5
- Don’t need an appointment
Test Format
- Some questions will be vocabish “word” questions and have “word answers”
- Some questions ask you if a situation is reasonable
The textbook
- The second edition
Labs do not meet this week
Attendance
- Using your clicker at all gets you half a point
- Using it correctly or luckily gets you the other half
Tests
- Need to bring an ID
- Labs can’t be programmable
There will not be recitation this evening
Chapter 1 - Atoms
Matter from the Particulate Point of View
- Matter is composed of particles
- Example: subatomic particles such as neutrons, protons, and electrons, atoms, and molecules
- “We divide the universe into two types of stuff”
- Example: subatomic particles such as neutrons, protons, and electrons, atoms, and molecules
- How the particles come together dictates the physical properties of matter
- How do things transform
- Matter is defined as anything that has mass and occupies space (e.g., has volume)
Elements, Molecules, and Mixtures: The Types of Matter
- Atoms
- Basic submicroscopic particles that constitute the fundamental building blocks of ordinary matter
- Molecules
- Substances formed when two or more atoms come together (bond) in specific geometric arrangements
- Atoms and molecules determine how matter behaves
- Chemistry is a discipline that seeks to understand matter and its properties, and the transformations that matter undergoes- particularly between molecules.
The Classification of Matter
- Matter can be classified according to
- its state — its physical form (i.e., solid, liquid, or gas) based on what properties it exhibits;
- its composition or the types of particles
- The state of matter changes from solid to liquid to gas with increasing temperature
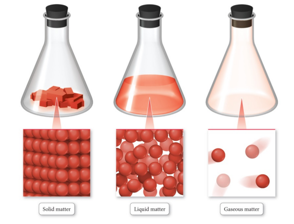
Solid Matter
- In solid matter, atoms or molecules pack close to each other in fixed locations.
- Although the atoms and molecules in a solid vibrate, they do not move around or past each other.
- Consequently, a solid has a fixed volume and
rigid shape.
- Ice, aluminum, and diamond are good examples of solids.
Liquid Matter
- In liquid matter, atoms or molecules pack about as closely as they do in solid matter, but they are free to move relative to each other.
- Liquids have fixed volume but not a fixed shape.
- Liquids’ ability to flow makes them assume the shape of their container.
- Water, alcohol, and gasoline are all substances that are liquids at room temperature.
Gaseous Matter
- In gaseous matter, atoms or molecules have a lot of space between them.
- They are free to move relative to one another.
- Fill available space
- These qualities make gases compressible.
Classification of Matter by Components
- Matter can be classified according to its composition: elements, compounds, and mixtures.
- a pure substance is made up of only one component, and its composition is invariant
- a mixture is a substance composed of two or more components in proportions that can vary from one sample to another

Vocab
| Term | Definition |
|---|---|
| matter | anything that has mass and occupies space (e.g. has volume) |
| atoms | Basic submicroscopic particles that constitute the fundamental building blocks of ordinary matter |
| molecules | Substances formed when two or more atoms come together (bond) in specific geometric arrangements |
| chemistry | a discipline that seeks to understand matter and its properties, and the transformations that matter undergoes- particularly between molecules |
| state | physical form of matter based on what properties it exhibits (i.e. solid, liquid, or gas. Classifies matter) |
| composition | Classifies matter based on the types of particles in it |
| solid matter | atoms and molecules in this type of matter pack close to each other in fixed locations |
| liquid matter | type of matter in which atoms or molecules are packed closely, but they are free to move relative to each other |
| gaseous matter | matter in which atoms or molecules have a lot of space between them |
| compressible | Material which are like gases are said to be… |
| pure substance | made up of only one component and its composition is invariant |
| mixture | substance composed of two or more components in proportions that can vary from one sample to another |