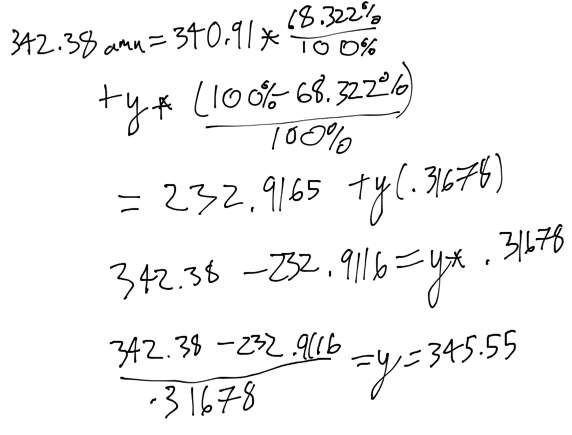Week 2 - Recitation
Questions
- How are gases different from solids and liquids?
- Gases can only be made up of atoms.
- The particles in a gas attract each other much more strongly than in solids and liquids
- Gases are compressible
- Gases are colorless
- Correctly report the result of the following computation
- 4
- 3.7
- 3.72
- 3.716
- 3.7162
- An atom which has lost an electron is
- a cation
- unlikely to be found in homogeneous mixtures
- electrically neutral
- likely to behave exactly like the parent atom
- an anion
- Determine the number of protons, neutrons and electrons in the following:
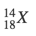
- p = 18, n = 18, e = 22
- p = 18, n = 22, e = 18
- p = 22, n = 18, e = 18
- p = 18, n = 22, e = 40
- p = 40, n = 22, e = 18
- Calculate the atomic mass of element “X” if it has 2 naturally occurring isotopes with the following masses and natural abundances
- X-107 106.90509 amu 51.84%
- X-109 108.90476 amu 48.46%
- 107.90 amu
- 108.00 amu
- 107.79 amu
- 108.32 amu
- 108.19 amu
- A new compound was recently discovered and found to have an atomic weight of 342.38 amu. This element has two isotopes, the lighter of which has a mass of 340.91 amu and an abundance of 68.322%. What is the mass of the heavier isotope?
- 350.21
- 345.55
- 348
- 108.32 amu
- 108.19 amu
- Two or more substances in variable proportions, where the composition is variable throughout are
- a solution
- a homogeneous mixture
- a compound
- an amorphous solution
- a heterogeneous mixture
- Calculate the mass of the air contained in a room that measures 2.50 m x 5.50 m x 3.00 m, given that the density of air is 1.29 g/cm^3 at 25 °C.
- 3.13 x 10^-5 g
- 32.0 kg
- 53.2 kg
- 53.2 g
- 32.0 g
-
Three students measure the density of copper (density of copper 8.92 g/cm^3) and obtain the following results:
Student A Student B Student C 7.99 g/cm^3 8.91 g/cm^3 6.50 g/cm^3 7.98 g/cm^3 8.90 g/cm^3 6.90 g/cm^3 8.01 g/cm^3 8.92 g/cm^3 7.20 g/cm^3
- Which student is precise but not accurate?
- Students A and B
- Student A
- Student C
- Student B
- Students B and C