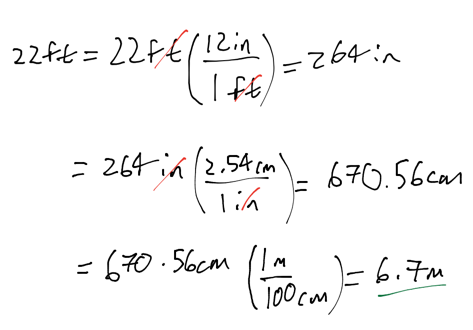Week 3 - Recitaion
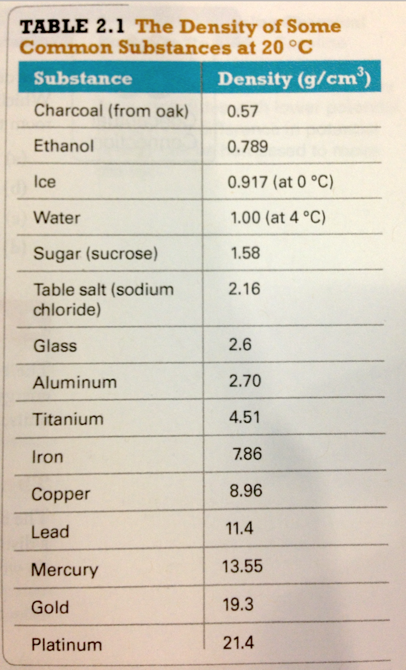
Density table
Questions
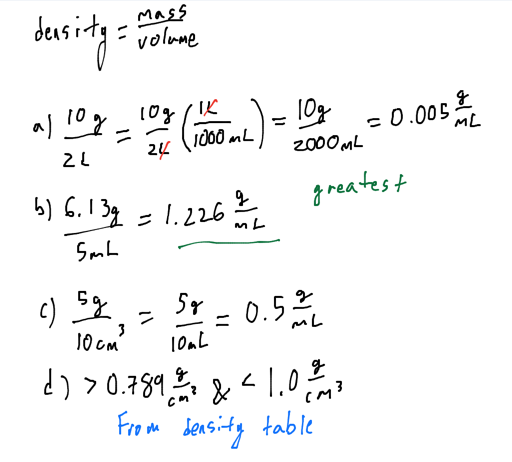
- Which of the following has the largest density?
- A material that has a mass of 10.0 g and a volume of 2.00 L
- A material that has a mass of 6.13 g and a volume of 5 mL
- A material that has a mass of 5 g and a volume of 10.0 cm^3
- A material that sinks in ethanol but floats in water
- A room measures 22 feet in width. What is the distance in meters? 1 in = 2.54 cm
- 72m
- 4.7m
- 1.0m
- 6.7m
- How many ounces of mercury are in 1.0 m^3 of mercury? Hint: 1 oz = 28.35 g.
- 6.5 * 10^6 oz
- 4.8 * 10^5 oz
- 5.2 * 10^4 oz
- 6.5 * 10^4 oz
- 48 oz
- Determine the mass of 2.5 cups of water if the density of water is 1.00 g/cm^3 and 1 cup = 240 mL
- 2.5 g
- 6.0 * 10^2
- 1.0 * 10^-2
- 2.4 * 10^2
- 1.0 * 10^2
- A cube of aluminum (density = 2.7 g/mL) has a mass of 17.2 g. What is the edge length of the cube?
- 6.34 cm
- 1.85 cm
- 2.58 cm
- 3.59 cm
- Which of the following is FALSE?
- The mole can be used to specify Avogadro’s number of anything
- Avogadro’s number, 6.022 * 10^23, is an exact number.
- The mole is equal to the number of atoms in exactly 12 g of carbon-12.
- The value of an element’s molar mass in grams per mole is numerically equal to the element’s atomic mass in amu.
- Place the following types of electromagnetic radiation in order of decreasing energy
- radio waves > infrared light > gamma rays
- gamma rays > infrared light > radio waves
- radio waves > gamma rays > infrared light
- gamma rays > radio waves > infrared light
- Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.88 * 10^14 Hz.
- 229 nm
- 436 nm
- 206 nm
- 485 nm
- 675 nm
- How many moles of Kr are contained in 398 mg of Kr?
- 4.75 * 10^-3 moles Kr
- 33.4 moles Kr
- 2.11 * 10^-4 moles Kr
- 2.99 * 10^-3 moles Kr
- 1.19 * 10^-4 moles Kr
- Calculate the mass (in kg) of 4.87 * 10^25 atoms of Zn
- 5.29 kg
- 1.89 kg
- 8.09 kg
- 1.24 kg
- 1.09 kg