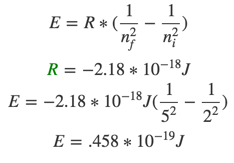Week 5 - Recitation (1st for test 2)
- Determine the energy change associated with the transition from n = 2 to n = 5 in the hydrogen atom.
- -2.18 * 10^-19 J
- +6.54*10^-19 J
- +4.58 * 10^-19 J
- -1.53 * 10^-19 J
- +3.76 * 10^-19 J
- It is possible to determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization energy (in kJ) for a mole of hydrogen atoms, making the assumption that ionization is the transition from n - 1 to n = infinity.
- 7.62 * 10^3 kJ
- 2.76 * 10^3 kJ
- 1.31 * 10^3 kJ
- 3.62 * 10^3 kJ
- 5.33 * 10^3 kJ
- Determine the end (final) value of n in a hydrogen atom transition, if the electron starts in n = 1 and the atom absorbs a photon of light with an energy of 2.044 * 10^-18 J
- 3
- 4
- 2
- 5
- 6
- How many different values of l are possible in the third principal level?
- 1
- 2
- 3
- 0
- 4
- Give the ground state electron configuration for Pb
- [Xe]6s2 6p2
- [Xe]6s2 5d10 6p2
- [Xe]6s2 5f14 6d10 6p2
- [Xe]6s2 4f14 5d10 6p2
- [Xe]6s2 4f14 5d10 6s2 6p2
- Choose the valence orbital diagram that represents the ground state of Zn

- A
- B
- C
- D
- E
- Give the possible values for ml for a p orbital
- 0, 1
- -1, 0, 1
- 1, 2
- -2, -1, 0, 1, 2
- Describe the shape of a p orbital
- spherical
- dumbbell shaped
- three lobes
- four lobes
- eight lobes
- No two electrons can have the same four quantum numbers is known as the
- Pauli exclusion principle
- Hund’s rule
- Aufbau principle
- Heisenberg uncertainty principle
- Choose the orbital diagram that represents the ground state of N
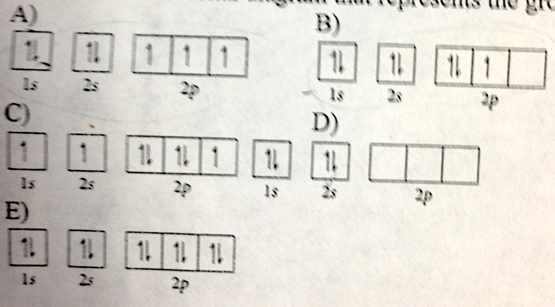
- A
- B
- C
- D
- E
- Predict the charge that a calcium ion would have
- 6-
- 2-
- 3+
- 2+
- 1+
- Which of the following statements is true?
- An orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy
- An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy
- It is possible for two electrons in the same atom to have identical values for all four quantum numbers
- Two electrons in the same orbital can have the same spin
- None of the above are true