Week 7 - Day 1
Navigate using audio
Mass Percent as a Conversion Factor
-
Audio 0:01:05.568971
- the mass percent tells you the mass of a constituent element in 100 g of the compound
- the fact that CCl2F2 is 58.64% Cl by mass means that 100 g of CCl2F2 contains 58.64 g Cl
- this can be used as a conversion factor
- 100 g CCl2F2 : 58.64 g Cl
Molecular Formulas
- Audio 0:01:54.462456
- The molecular formula is a multiple of the empirical formula
- Molecular Mass is the same multiple of the mass of the empirical formula
- Benzene:
- Empirical Formula: CH
- 13.02 amu, 13.02 g/mol
- Molecular Formula: C6H6 which is 6xCH
- 78.11 amu, 78.11 g/mol
Determining a Chemical Formula from Experimental Data
- Audio 0:03:56.552943 Empirical Formula:
- Simplest whole-number ratio of the atoms of elements in a compound
- Can be determined from elemental analysis
- Masses of elements formed when a compound is decomposed, or that react together to form a compound
- Combustion analysis
- (Mass) Percent composition Note: An empirical formula represents a ratio of atoms or a ratio of moles of atoms, not a ratio of masses.
Finding an Empirical Formula
- Audio 0:05:56.439211
- Convert the percentages to grams.
- a) If not given, assume you start with 100 g of the compound.
- b) Example: 24.5% C means 24.5 g C.
- Convert mass (grams) to moles. a) Use molar mass of each element. b) Example: 24.5 g C × (1 mol C/12.01 grams) = 2.00 mol C
- Divide all by the smallest number of moles to obtain the atom- to-atom ratio for each of the elements in the compound. a) If the result is within 0.1 of a whole number, round to the whole number.
- Multiply all mole ratios by a number to make all whole numbers. a) If ratio is .5, multiply all by 2; if the ratio is .33 or .67, multiply all by 3; and so on. b) Skip if already whole numbers.
- Convert the percentages to grams.
Example
- What is the empirical formula of a substance that contains 5.28 g of C, 1.11 g of H, and 3.52 g of O?
A) C2H5O B) C2H4O2 C) C2H4O3 D) C3H4O4
- Answer is A
- Audio 0:11:13.430592
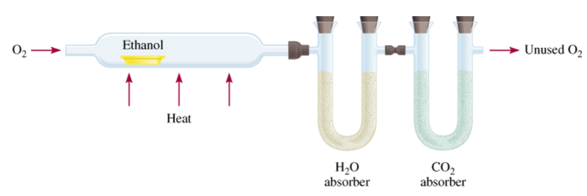
Combustion Analysis
- A common technique for analyzing compounds is to burn a known mass of compound and weigh the amounts of product made.
- This is generally used for organic compounds containing C, H, or O.
- Audio 0:22:35.779419
-
Ex: you combust 11.5 g ethanol and collect 22.0 g CO2 and 13.5 g H2O.
- By knowing the mass of the product and composition of constituent element in the product, the original amount of constituent element can be determined.
- All the original C forms CO2, the original H forms H2O, and the original mass of O is found by subtraction.
- Once the masses of all the constituent elements in the original compound have been determined, the empirical formula can be found.
Clicker
what is the empirical formula of the compound with the following composition? 2.1 % H, 65.3 % O, 32.6% S (H: 1.008, O: 16, S: 32.06) + C2H5O
Example 5.16 + Find the molecular formula of butanedione
- Given: emp. form. = C2H3O; MM = 86.09 g/mol
- Find: molecular formula
- C4H6O2
Combustion analysis clicker
- Audio 0:34:34.156694

Carbon Bonding
- Audio 0:39:10.776833
- carbon atoms bond almost exclusively covalently
- compounds with ionic bonding C are generally inorganic
- when C bonds, it forms 4 covalent bonds
- 4 single bonds, 2 double bonds, 1 triple + 1 single, etc.
- carbon can form limitless chains of C atoms, both straight and branched, and rings of C atoms
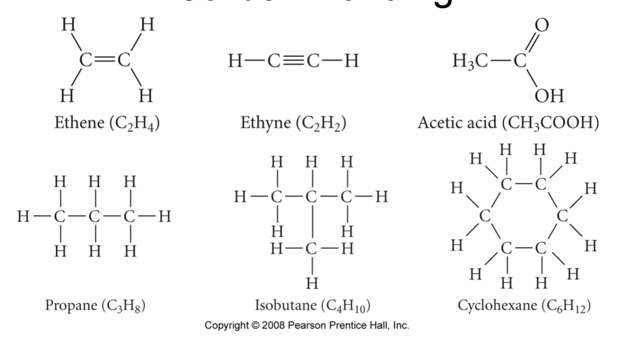
Classifying Organic Compounds
- there are two main categories of organic compounds, hydrocarbons and functionalized hydrocarbons
- hydrocarbons contain only C and H
- most fuels are mixtures of hydrocarbons

Classifying Hydrocarbons
- Audio 0:41:37.320899
- hydrocarbons containing only single bonds are called alkanes
- hydrocarbons containing one or more C=C are called alkenes
- hydrocarbons containing one or more C=C are called alkynes
- hydrocarbons containing C6 “benzene” ring are called aromatic
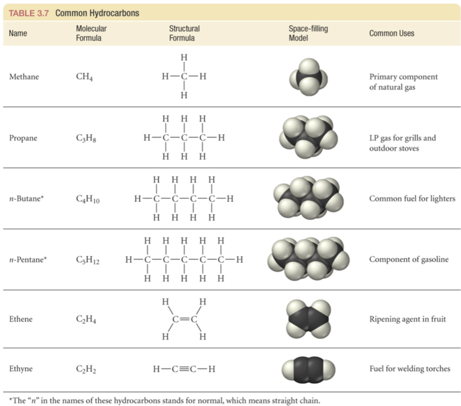
Naming Straight Chain Hydrocarbons
- Base name to indicate the number of carbons in the chain
- suffix to indicate class and position of multiple bonds suffix –ane for alkane, –ene for alkene, –yne for alkyne
-