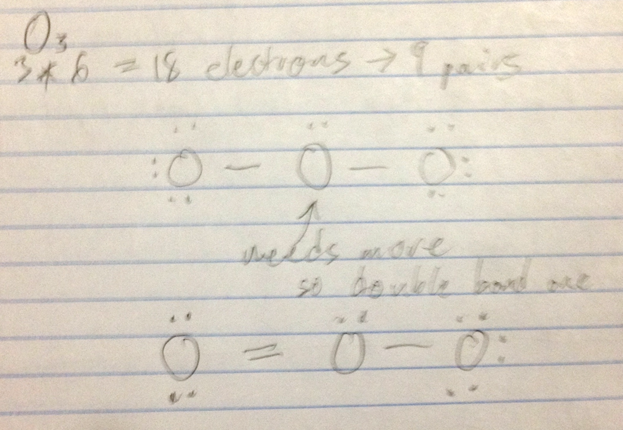Week 7 - Day 2
Navigate using audio
Announcements
- No recitation tonight
Ch 6 - Lecture 1 (Chemical Bonding I)
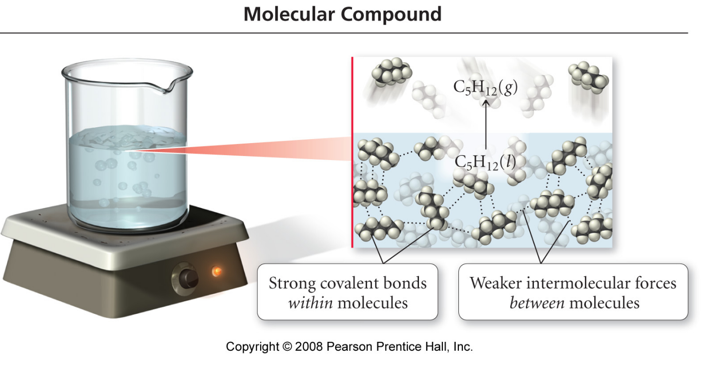
Covalent Bonding Model vs. Reality
- Audio 0:01:17.116824
- molecular compounds have low melting points and boiling
points
- MP generally < 300°C
- molecular compounds are found in all 3 states at room temperature
- melting and boiling involve breaking the attractions
between the molecules, but not the bonds between the
atoms
- the covalent bonds are strong
- the attractions between the molecules are generally weak
- the polarity of the covalent bonds influences the strength of the intermolecular attractions
Intermolecular Attractions vs. Bonding
Covalent Bonding Model vs. Reality
- Audio 0:03:16.295803
- some molecular solids are brittle and hard, but many are soft and waxy
- the kind and strength of the intermolecular attractions varies based on many factors
- the covalent bonds are not broken molecular compounds do not conduct electricity in the liquid state
- molecular acids conduct electricity when dissolved in water, but not in the solid state
- in molecular solids, there are no charged particles around to allow the material to conduct
-
when dissolved in water, molecular acids are ionized, and have the ability to move through the structure and therefore conduct electricity
- Audio 0:04:17.779258
Polar Covalent Bonding
- Audio 0:05:06.884522
- Covalent bonding between unlike atoms results in unequal sharing of the electrons.
- One atom pulls the electrons in the bond closer to its side.
- One end of the bond has larger electron density than the other.
- The result is a polar covalent bond.
- Bond polarity
- The end with the larger electron density gets a partial negative charge.
- The end that is electron deficient gets a partial positive charge.
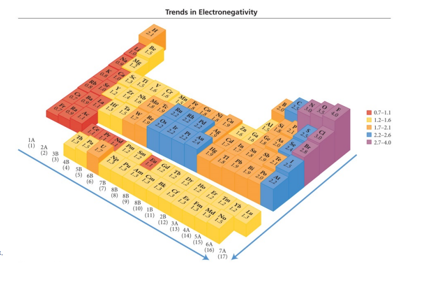
Electronegativity (EN)
- Audio 0:06:39.736104
- A measure of an elements ability to pull electrons toward it
- An empirical scale drawn from measured properties of many molecules
- provides “rule of thumb” to predict molecular properties
Electronegativity
- Audio 0:07:30.692317
- The ability of an atom to attract bonding electrons to itself is called electronegativity.
- An empirical scale drawn from measured properties of many molecules
- provides “rule of thumb” to predict molecular properties
- The larger the difference in electronegativity, the more polar the bond.
- Negative end toward more electronegative atom
- Audio 0:07:45.217060
- Scale defined so Fluorine is the most electronegative, Francium the least
- Increases across a period (left to right) and decreases
down a group (top to bottom)
- Noble gas atoms are not assigned EN values.
- Opposite of atomic size trend
- Audio 0:09:56.193372
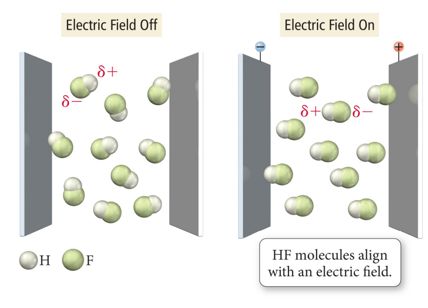
Electronegativity Example
- Audio 0:11:11.916765
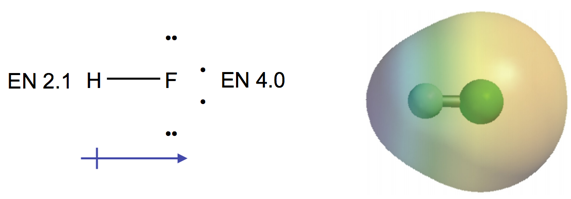
Bond Dipole Moments
- Audio 0:11:29.357496
- Dipole moment, µ, is a measure of bond polarity.
- A dipole is a material with a + and − end.
- It is directly proportional to the size of the partial charges (q) and directly proportional to the distance (r) between them.
- µ (dipole moment) = (q)(r)
- Measured in Debyes, D
- Generally, the more electrons two atoms share and the larger the atoms are, the larger the dipole moment.

Electronegativity Difference and Bond Type
- Audio 0:13:12.370573
- If the difference in electronegativity between bonded
atoms is 0, the bond is pure covalent.
- Equal sharing of the atoms in the bond
- If the difference in electronegativity between bonded atoms is 0.1 to 0.4, the bond is nonpolar covalent.
- If the difference in electronegativity between bonded
atoms is 0.5 to 1.9, the bond is polar covalent.
- Unequal sharing of electrons between the atoms in the bond
- If the difference in electronegativity between bonded atoms is larger than or equal to 2.0, the bond is ionic.
Percent Ionic Character
- Audio 0:15:43.076694
- The percent ionic character is the percentage of a bond’s measured dipole moment compared to what it would be if the electrons were completely transferred.
- The percent ionic character indicates the degree to which the electron is transferred.

Molecular Structure I: Octet Rule
- Audio 0:16:44.675983
- Generally eight electrons in valence shell
- exceptions for H, Li, (2 electrons) and Be and B (only 2 and 3 electrons)
- “Octet Expansion”: S, P, etc where can have more than 8 electrons when central atom
Writing Lewis Structures for Molecular Compounds
- Audio 0:17:58.111765
- Write the correct skeletal structure for the molecule.
- The less electronegative atom in the molecule is usually the center atom.
- Simple molecules have a “center” atom to which all of the other atoms in the molecules are attached (bonded) to.
- For example, for CO2, the center atom is C, so both O atoms are attached to the carbon atom.
- Audio 0:18:55.177204
- O–C–O
- For example, for CO2, the center atom is C, so both O atoms are attached to the carbon atom.
- Simple molecules have a “center” atom to which all of the other atoms in the molecules are attached (bonded) to.
- The more electronegative atoms are usually terminal (attached to the center atom).
- Hydrogen atoms are always in the terminal position.
- The less electronegative atom in the molecule is usually the center atom.
- Determine the total number of valence electrons each atom brings to form the
molecule.
- Examples:
- For the molecule H–Br: H atom brings 1 electron and Br atoms brings 7 electrons for a total 8 electrons
- In polyatomic ions, the charge on the ion also must be accounted. For the polyatomic ion NO2 –, a total of 18 electrons are brought in: 5 electrons from N, total 12 from O (2 oxygen atoms × 2), and 2 from the 2+ charge.
- Examples:
Writing Lewis Structures for Molecular Compounds
- Audio 0:20:36.268038
- Distribute the electrons among the atoms in the molecule giving octets (or duets in the case of hydrogen) to as many atoms as possible.
- The best practice is to place two electrons around an atom at a time.
- Bonding pairs: electrons between two atoms
- Nonbonding or lone pairs: electrons not participating in bonding but complete the atom’s “octet”
- The total number of electrons brought in must be accounted for in the Lewis structure and must not violate any criteria (i.e., H atoms can only have single bonds or two electrons total).
- The best practice is to place two electrons around an atom at a time.
- If any atoms lack an octet, form double or triple bonds as necessary to give
them octets.
- Atoms that can multiple bond with each other or to themselves are as follows:
- Double bond (4 electrons or two pairs of electrons between atoms): C, O, N, S, & P
- Triple bond (6 electrons or three pairs of electrons between atoms): C,O, N, & S
- Atoms that can multiple bond with each other or to themselves are as follows:
Practice Problem Writing Lewis Structures for Polyatomic Ions
NH4+
- Audio 0:23:57.666020
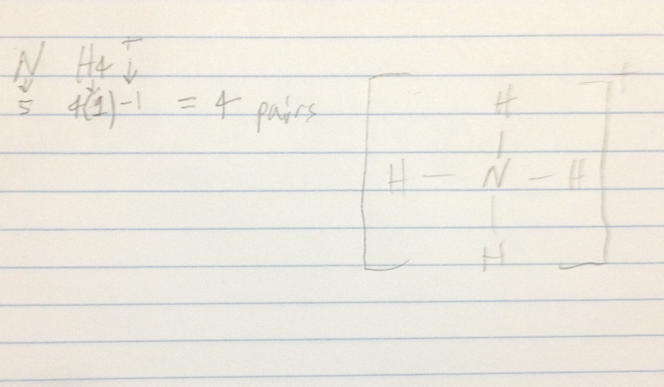
O3
Resonance and Formal Charges
- Audio 0:31:22.898676
- Two additional concepts to write the best possible Lewis structures for a large number of compounds
- The concepts are:
- Resonance, used when two or more valid Lewis structures can be drawn for the same compound
- Formal charge, an electron bookkeeping system that allows us to discriminate between alternative Lewis structures
Resonance
- Audio 0:32:45.308690
- Lewis theory localizes the electrons between the atoms that are bonding together.
- Extensions of Lewis theory suggest that there is some degree of
delocalization of the electrons; the concept is called resonance.
- Delocalization of charge helps to stabilize the molecule.
- When there is more than one Lewis structure for a molecule that differ only in the position of the electrons, they are called resonance structures.
- The actual molecule is a combination of the resonance forms—a resonance hybrid.
- The molecule does not resonate between the two forms, though we often draw it that way.
- Example: O3 molecule
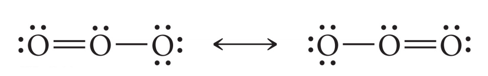

- Resonance hybrid: Just as the offspring of two different dog breeds is a hybrid that is intermediate between the two breeds (a), the structure of a resonance hybrid is intermediate between that of the contributing resonance structures (b).
Ex
- Audio 0:38:19.039491

Clicker
- Audio 0:38:59.721909
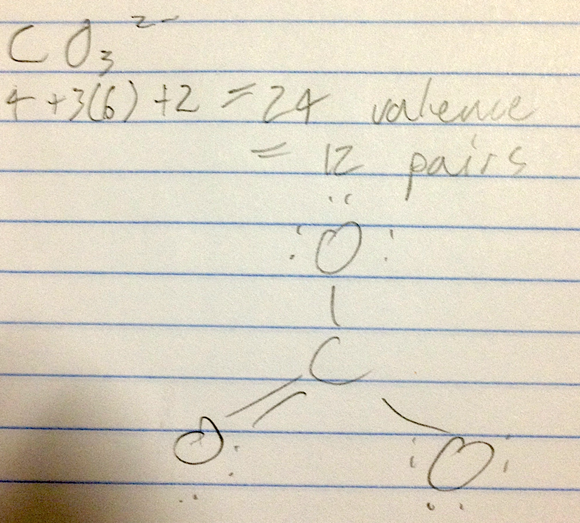
- Draw the Lewis structure for CO3^2- including any valid resonance structures.
- two single bonds and one double bond
Formal Charge
- Audio 0:42:50.124155
- Are all Resonance Structures equally good?
- NO
- so how do we decide which to give more weight?
- Audio 0:43:41.519126
- charge an atom would have if all bonding electrons are shared equally between the bonded atoms.
{(# of valence electrons) + (# of nonbonding electrons)- (1/2 × # of bonding electrons)}
- The sum of all formal charges in a neutral molecule must be zero.
- The sum of all formal charges in an ion must equal the charge of the ion.
- Small (or zero) formal charges on individual atoms are better than large ones.
- When formal charge cannot be avoided, negative formal charge should reside on the most electronegative atom.
- Audio 0:44:42.818811
- Example: The molecule HF has 0 (zero) formal charge.
- The formal charge on H atom: Formal charge = 1 − [0 + ½ (2)] = 0
- The formal charge on F atom: Formal charge = 7 − [6 + ½ (2)] = 0
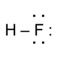
Vocab
| Term | Definition |
|---|---|
| empirical formula | simplest whole-number ratio of the atoms of elements in a compound |
| molecular compounds have _ melting points and boiling points | low |
| polar covalent bond | results from covalent bonding between unlike atoms results in unequal sharing of the electrons |
| electronegativity | the ability of an atom to attract bonding electrons to itself |
| electronegativity _ across a period | increases left to right |
| electronegativity _ down a group | decreases |
| dipole moment (µ) | measure of bond polarity (= q * r) |
| pure covalent | the bond is this when the difference in electronegativity between bonded atoms is 0 |
| nonpolar covalent | if the difference in electronegativity between bonded atoms is 0.1 to 0.4, the bond is |
| percent ionic character | the percentage of a bond’s measured dipole moment compared to what it would be if the electrons were completely transferred |
| resonance | when two or more valid Lewis structures can be drawn for the same compound |
| formal charge | an electron bookkeeping system that allows us to discriminate between alternative Lewis structures |
| resonance structures | when there is more than one Lewis structure for a molecule that differ only in the position of the electrons |
| formal charge | charge an atom would have if all bonding electrons are shared equally between the bonded atoms |