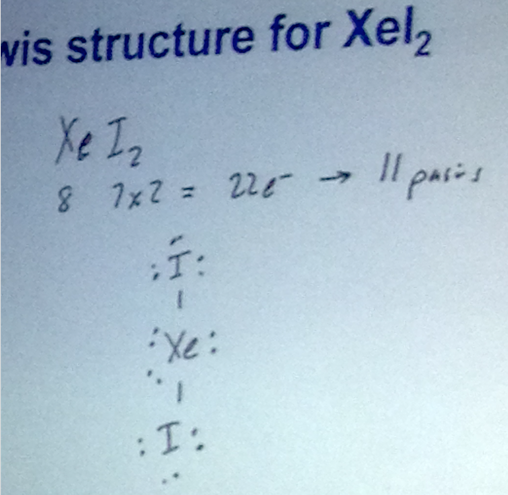Week 7 - Day 3 (Ch 6 - pt 2)
Navigate using audio
Announcements
- Left off on formal charge
- Test 2 next Wednesday
- Chapters 4 - 6
- Just where we left off
- Same rules as last time
- Bring photo id
- bring pencil
- non-programmable calculator
- Chapters 4 - 6
Clicker 1
Ch 6 continued (pt 2)
Example: Formal Charge, SO2
- Audio 0:06:38.049121

- Formal charges: located on “appropriate atoms”
- Audio 0:10:37.025317
- Per atom, to calculate formal charge, you take the valence electrons minus the electrons on it minus the number of pairs on it.
Practice Problem Assigning Formal Charges
- Audio 0:12:40.335303
- OCN-

Rules of Resonance Structures
- Audio 0:19:11.165662
- Resonance structures must have the same connectivity.
- Only electron positions can change.
- Resonance structures must have the same number of electrons.
- Second row elements have a maximum of eight electrons.
- Bonding and nonbonding
- Third row can have expanded octet
- Formal charges must total the same.
- Better structures have fewer formal charges.
- Better structures have smaller formal charges.
- Better structures have the negative formal charge on the more electronegative atom.
Expanded Octets, Odd-Electron, and Other Species: The Exceptions to the Octet “Rule”
- Audio 0:21:06.805441
- The exceptions:
- Expanded octets:
- Molecules or ions with more than eight electrons around an atom
- Involve the nonmetal elements located in the 3rd period and below
- Nonmetals (3rd period down in the periodic table) follow the
octet rule when they are not the “center” atom.
- The center atom is the atom in the molecule where the other elements individually bond to (attach).
- When they are the center atom, they can accommodate more than eight electrons.
- Using empty valence d orbitals that are predicted by quantum theory
- Odd-electron species (free radicals or radicals):
- Molecules or ions with an odd number of electrons
- Legitimate Lewis structures cannot be written for they do not meet the “octet rule” as required by the Lewis model.
- Example: NO
- Has 11 valence electrons
- Distribution of 11 electrons cannot meet the criteria under the Lewis model.
- NO does exist as a molecule.
- The Lewis model is not sophisticated enough to work for an odd number of electron compounds.
- Audio 0:22:20.060270
- Incomplete octets:
- Elements (specifically metalloids and H atom) whose tendency is not to have a complete octet
- H can only accompany two electrons (duet).
- Boron (metalloid)
- Prefer 6 electrons than 8 electrons
- Elements (specifically metalloids and H atom) whose tendency is not to have a complete octet

Clicker 2
- What is the formal charge on the sulfur for best structure for the sulfate anion, SO4^2-?
- Audio 0:27:21.796062

- Most people said plus 2, but the answer is actually 0
Bond Energies
- Audio 0:32:36.686918
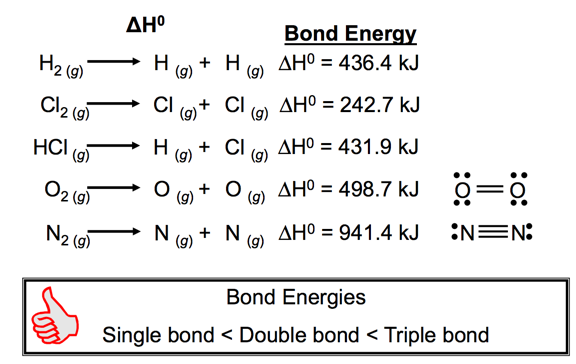
- Chemical reactions involve breaking bonds in reactant molecules and making new bonds to create the products.
- The change in energy for a reaction can be estimated by comparing the cost of breaking old bonds to the energy released from making new bonds.
- The amount of energy, in the gaseous state, that it takes to break one mole of a bond in a compound is called the bond energy.
- Audio 0:34:03.982413
- The energy change required to break a particular bond in one mole of gaseous molecules is the bond energy
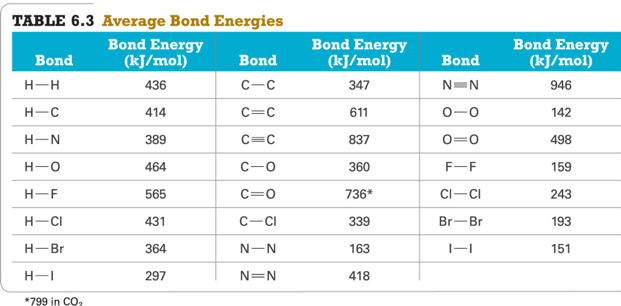
Trends in Bond Energies
- Audio 0:36:20.771621
- In general, the more electrons two atoms share, the
stronger the covalent bond.
- For comparison of bonds between like atoms
- C≡C (837 kJ) > C═C (611 kJ) > C—C (347 kJ)
- C≡N (891 kJ) > C ═ N (615 kJ) > C—N (305 kJ)
- In general, the shorter the covalent bond, the stronger
the bond.
- For comparison of bonds between like atoms
- Br—F (237 kJ) > Br—Cl (218 kJ) > Br—Br (193 kJ)
- Bonds get weaker down the column.
- Bonds get stronger across the period.
Average Bond Energies
Covalent Bonding: Model versus Reality for Bond Strength
- Audio 0:39:20.498710
- Lewis theory predicts that the more electrons two atoms
share, the stronger the bond.
- Single bond < Double bond < Triple bond
- Lewis theory would predict that double bonds are twice as strong as single bonds, but the reality is they are less than twice as strong.
- Bond strength is measured by how much energy must be added into the bond to break it in half.
Covalent Bonding: Model versus Reality for Bond Length
- Audio 0:40:57.263317
- Lewis theory predicts that the more electrons two atoms share, the shorter the bond should be.
- When comparing bonds to like atoms
- Bond length is determined by measuring the distance between the nuclei of bonded atoms.
- In general, triple bonds are shorter than double bonds, and double bonds are shorter than single bonds.
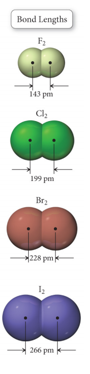
Bond Lengths
- Audio 0:42:20.387950
- The distance between the nuclei of bonded atoms is called the bond length.
- Because the actual bond length depends on the other
atoms around the bond, we often use the average
bond length.
- Averaged for similar bonds from many compounds

Vocab
| Term | Definition |
|---|---|
| bond energy | the amount of energy, in the gaseous state, that it takes to break one mole of a bond in a compound |
| bond strength | measured by how much energy must be added into the bond to break it in half |
| bond length | determined by measuring the distance between the nuclei of bonded atoms |
| as bonds get longer they get _ | weaker |
| bonds get _ down a column and _ across a period | weaker / stronger |