Week 8 - Day 2 (Last before test 2)
Navigate using audio
Announcements
- Audio 0:00:32.424965
- Test tonight
- Bring pencil, photo id, calculator
- You can start as late as 7 and you can’t leave until 7
- Material from end of chapter 3 to end of chapter 6
- Coulomb’s Law is physics
- Only expects you to understand the directions / relative magnitudes of charges (no calculations)
Clicker 1
- Audio 0:05:31.262428
- According to VSEPR theory, which one of the following molecules should have a molecular geometry that is trigonal bipyramidal?
- SF4
- XeF4
- NF3
- SF6
- PF5
- The answer is PF5 because it is the only answer with five atoms attached to the central atom
Representing Three-Dimensional Shapes on Paper
- Audio 0:07:41.096639
- Drawing molecules to show their dimensionality on paper (2-D) is difficult.
- How to draw a 3-D representation of molecule on paper:
- By convention, the central atom is put in the plane of the paper.
- Put as many other atoms as possible in the same plane and indicate with a straight line.
- For atoms in front of the plane, use a solid wedge.
- For atoms behind the plane, use a hashed wedge

Illustrations of Molecular Geometries of Molecules Using 3-D Notations
- Audio 0:10:13.039414
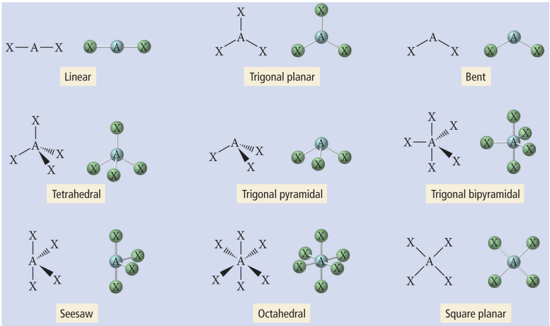
Multiple Central Atoms and Their Geometries
- Audio 0:11:15.077734
- Many molecules have larger structures with many interior atoms.
- Looking at “multiple center atoms”
- Think of them as having multiple central atoms.
- For multiple center molecules:
- Each center atom has a designated a shape.
- Example: Glycine
- The shape around the:
- N atom is trigonal pyramidal
- Left C is tetrahedral
- Right C is trigonal planar
- O is bent

Clicker 2
- Audio 0:15:17.202864
- Which species has the highest ionization energy?
- A) Mg
- B) Mg+
- C) Mg2+
- D) Al+
- E) Al2+
Clicker 3
- Audio 0:16:45.881866
- Give the ground state electron configuration for the ion of Ba
- [Kr] 5s24d105p6
Clicker 4
- Audio 0:19:07.805288
- Which reaction below represents the electron affinity of Li?
- Answer in Recitation questions
Clicker 5
- Audio 0:20:44.692817
- a) Na+
- b) Ga3+
- c) K+
- d) Mg2+
- e) Ca2+
- C
Clicker 6
- Audio 0:23:43.818176
- a) titanium (II) carbonate
- b) titanium carbide
- c) titanium carbonite
- c) titanium (II) carbonite
- a
Clicker 7
- Audio 0:26:11.549016
- Calculate the molar mass of Al(C2H3O2)3
- a) 86.03 g/mol
- b) 204.13 g/mol
- c) 56.00 g/mol
- d) 258.09 g/mol
- e) 139.9 g/mol
- B
Clicker 8
- Audio 0:29:32.460732
- How many atoms of oxygen are contained in 47.6 g of Al2(CO3)3? The molar mass of Al2(CO3)3 is 233.99 g/mol
- We have 1/5th of a mol of Al2(CO3)3. There are nine mols of oxygen in one molecule. = 9 * avogandros number
Clicker 9
- Audio 0:33:28.381248
- Determine the empirical formula for a compound that is 36.86% N and 63.14% O by mass. (O:15.999, N:14.0007)
- N2O3
Clicker 10
- Audio 0:34:36.281792
- Determine the molecular formula of a compound that is 49.48% carbon, 5.19% hydrogen,28.85% nitrogen, and 16.48% oxygen. The molecular weight is 194.19 g/mol
Clicker 11
- Audio 0:38:58.435866
- Which of the following reactions is associated with the lattice energy of CaS?
- Ca(s) + S(s) -> CaS(s)
- CaS(s) + S(s) -> CaS(s)
- Ca2-(aq) + S2-(aq) -> CaS(s)
- Ca2-(g) + S2-(g) -> CaS(s)
- D
Clicker 11
- Choose the bond below that is the least polar
- P-F
- C-Br
- C-F
- C-I
- C-Cl
- Want to find the least electronegative difference
- D
Clicker 12
- Which of the following resonance structures for OCN- will contribute most to the correct structure of OCN-?
- A) O(3 lone pairs) - C N(with 1 lone pair)
Vocab
| Term | Definition |
|---|---|
| solid wedge | 3D representation for drawing molecular structures which represents a bond coming out of the page |
| solid hatched | 3D representation for drawing molecular structures which represents a bond going into the page |
| straight line | representation for drawing molecular structures which represents a bond in plane of the drawing surface |