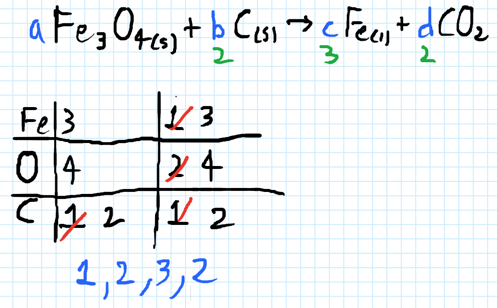Recitation Week 10 (test 3 - Recitation 2)
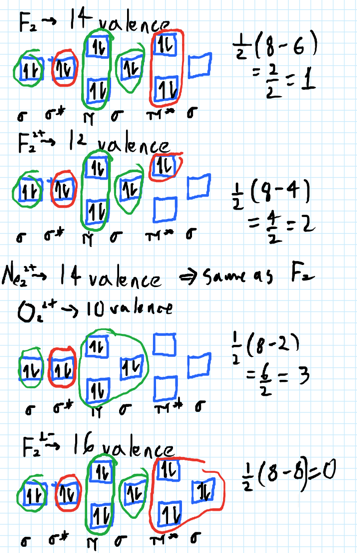
- 1) Draw the molecular orbital diagrams to determine which of the following is most stable.
- A) F2
- B) F2^2+
- C) Ne2^2+
- D) O2^2+
- E) F2^2-
- 2) Use molecular orbital diagrams to determine which of the following are paramagnetic.
- A) O2^2-
- B) Ne2^2+
- C) O2^2+
- D) F2^2+
- E) None of the above are paramagnetic
- 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic.
- A) B2^2+
- B) B2^2-
- C) N2^2+
- D) C2^2-
- E) B2
- 4) Draw the molecular orbital diagram shown to determine which of the following is most stable.
- A) C2^2+
- B) N2^2+
- C) B2
- D) C2^2-
- E) B2^2+
- 5) Which statement regarding stable heteronuclear diatomic molecules is false?
- A) All have bond orders greater than zero.
- B) The antibonding molecular orbitals have more of the character of the more electropositive element than of the more electronegative element.
- C) Their molecular orbital diagrams are more symmetrical than those of homonuclear diatomic molecules.
- D) The bonding molecular orbitals have more of the character of the more electronegative element than of the less electronegative element.
- E) The greater is the difference in energy between two overlapping atomic orbitals, the more polar the resulting bond is, due to electrons occupying the resulting bonding molecular orbital.
- 6) When the equation below is correctly balanced, the coefficients a, b, c, d have which values?
- a Fe3O4(s) + b C(s) -> c Fe(l) + d CO2(g)
- A) 1232
- B) 1321
- C) 2121
- D) 2132
- 7) How many grams of Li3N can be formed from 1.75 moles of Li? Assume an excess of nitrogen.
- 6 Li(s) + N2(g) -> 2 Li3N(s)
- A) 18.3 g Li3N
- B) 20.3 g Li3N
- C) 58.3 g Li3N
- D) 61.0 g Li3N
- E) 15.1 g Li3N
- 8) A 14.01 g sample of N2 reacts with 3.02 g of H2 to form ammonia (NH3). If ammonia is the only product, what mass of ammonia is formed?
- A) 17.03 g
- B) 1.10 g
- C) 14.01 g
- D) 3.02 g
- E) 23.07 g
- 9) Consider the following balanced reaction. How many grams of water are required to form 75.9 g of HNO3? Assume that there is excess NO2 present. The molar masses are as follows: H2O = 18.02 g/mol, HNO3 = 63.02 g/mol.
- 3 NO2(g) + H2O(l) -> 2 HNO3(aq) + NO(g)
- A) 38.0 g H20
- B) 21.7 g H20
- C) 43.4 g H20
- D) 10.9 g H20
- E) 26.5 g H2O
- 10) Give the theoretical yield, in moles, of CO2 from the reaction of 4.00 moles of C8H18 with 4.00 moles of 02.
- 2C8H18 + 25O2 -> 16CO2 + 18H2O
- A) 0.640 moles
- B) 64.0 moles
- C) 2.56 moles
- D) 16.0 moles
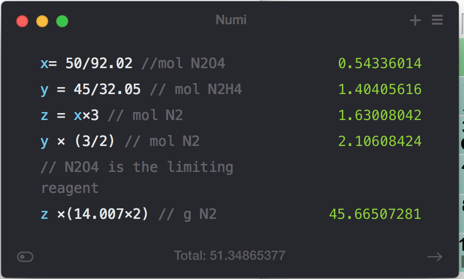
- 11) Determine the limiting reactant (LR) and the mass (in g) of nitrogen that can be formed from 50.0 g N204 and 45.0 g N2H4. Some possibly useful molar masses are as follows: N204 = 92.02 g/mol, N2H4 = 32.05 g/mol.
- N204(1) + 2 N2H4(1) 3 N2(g) + 4 H20(g)
- A) LR = N2H4, 59.0 g N2 formed
- B) LR = N2O4, 105 g N2 formed
- C) LR = N2O4, 45.7 g N2 formed
- D) LR = N2H4, 13.3 g N2 formed
- E) No LR, 45.0 g N2 formed
- 12) Give the percent yield when 28.16 g of CO2 are formed from the reaction of 4.000 moles of QH18 with 4.000 moles of O2.
- 2 C8H18 + 25 O2 -> 16 CO2 + 18 H2O
- A) 20.00%
- B) 25.00%
- C) 50.00%
- D) 12.50%