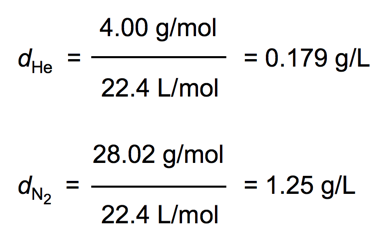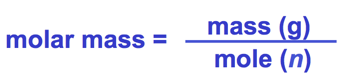Week 16 - Day 1
Navigate using audio
Final
- Week from today
- Slightly more weighted toward things on ch 10 and 11
- Review session
- 2 - 4:30 Saturday and Sunday after next
- review sessions in Shelby hall
- 2 - 4:30 Saturday and Sunday after next
-
Final is 11:30 - 2
- Liquid nitrogen experiment
Clicker 1
- Audio 0:06:15.196390
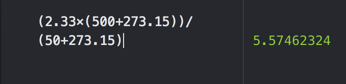
- To what volume will a sample of gas expand if it is heated from 50.0 C and 2.33 L to 500.0 C?
- A) 5.57 L
- B) 23.3 L
- C) 0.233 L
- D) 0.97 L
- E) 0.184 L
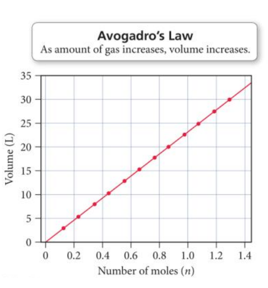
Avogadroʼs Law: Volume and Moles Have a Direct Relationship
- Audio 0:11:31.193983
- Volume is directly proportional to the number of gas molecules when pressure and temperature are held constant. – More gas molecules = larger volume
- Equal volumes of gases contain equal numbers of molecules. – The gas doesn’t matter.
- V = constant × n (moles)
- V/n = constant
- (V1/n1) = (V2/n2)
- The volume of a gas sample increases linearly with the number of moles of gas in the sample.
Clicker 2
- Audio 0:14:23.279569
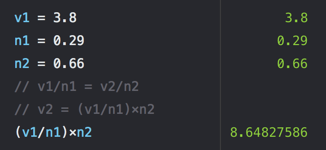
- If a sample of 0.29 moles of Ar occupies 3.8 L under certain conditions, what volume will 0.66 moles occupy under the same conditions?
- A) 12
- B) 8.6
- C) 17
- D) 5.0
- E) 15
Ideal Gas Law: PV = nRT
- Audio 0:17:10.738500
- The simple gas law relationships discussed so far can be combined into a single law that encompasses all of them.
- V α (1/P) Boyle’s Law
- V α T Charles’s Law
- V α n Avogadro’s Law
- Ideal gas law: PV = nRT
– Where
- P is pressure in atm
- V is volume in liters
- n is moles
- R is the ideal gas law constant, 0.0821 (L · atm)/(K · mol) – T is temperature in kelvins
Ideal Gas Law: PV = nRT
- Audio 0:18:31.572724
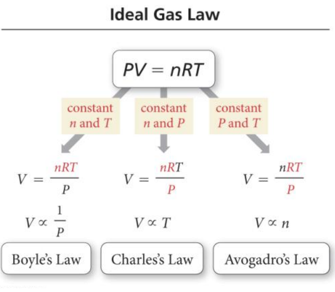 · The other gas laws are found in the ideal gas law if two variables are kept constant.
· The ideal gas law allows us to find one of the variables if we know the other three.
· The other gas laws are found in the ideal gas law if two variables are kept constant.
· The ideal gas law allows us to find one of the variables if we know the other three.
Practice Problem: Ideal Gas Law
- Audio 0:19:52.714114
- Calculate the volume occupied by 0.845 mol of nitrogen gas at a pressure of 1.37 atm and temperature of 42 °C
Practice Problem: Ideal Gas Law
-
Audio 0:22:41.752192
-
Calculate the number of moles of gas in a 3.24 L basketball inflated to a total pressure of 24.3 psi
Standard Conditions
- Audio 0:26:03.240956
- Because the volume of a gas varies with pressure and temperature, chemists have agreed on a set of conditions to report our measurements so that comparison is easy. – These are called standard conditions (STP).
- Standard pressure = 1 atm
- Standard temperature = 273 K = 0 °C
- Standard amount = 1 mol
- Standard volume = 22.4 L – The volume occupied by one mole of a substance is its molar volume at STP (T = 273 K or 0 °C and P = 1atm).
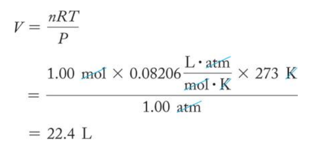
Molar Volume at STP
- Audio 0:28:15.214651
- The volume of one mole of gas at STP is called the molar volume.
- 6.022 × 1023 molecules of gas – Note that the type of gas is immaterial.
- It is important to recognize that one-mole measures of different gases have different masses, even though they have the same volume.
Density of a Gas
- Audio 0:29:06.988437
- Density is the ratio of mass to volume. – Density = (mass/volume)
- Density of a gas is generally given in grams/liter (g/L).
- The mass of 1 mol = molar mass.
- The volume of 1 mol at STP = 22.4 L.
- Density (d) = [mass of gas (g/mol)]/[volume (L)]
- Density (g/L) = (molar mass)/(molar volume)
- Density (d) = [mass of gas (g/mol)]/[volume (L)]
Density of a Gas at STP
- Audio 0:29:33.594188
- For example, the densities of helium and nitrogen gas at STP are as follows:
Molar Mass of a Gas
- Audio 0:30:27.999878
- One of the methods chemists use to determine the molar mass of an unknown substance is to heat a weighed sample until it becomes a gas; measure the temperature, pressure, and volume; and use the ideal gas law.
Gas Density
- Audio 0:31:34.143033
- PV = nRT
Gas Density
- Audio 0:34:08.137349

Practice Problem: Density of a Gas
- Audio 0:34:25.383706
- A sample of gas has a mass of 0.311 g. Its volume is 0.225 L at 55 oC and pressure of 886 mmHg. What is the molar mass?
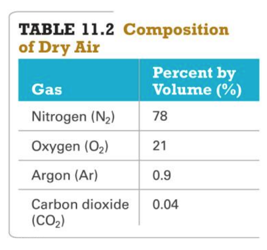
Mixtures of Gases and Partial Pressures
- Many gas samples are not pure but are mixtures of gases.
- Dry air, for example, is a mixture containing nitrogen, oxygen, argon, carbon dioxide, and a few other gases in trace amounts.
- Therefore, in certain applications, the mixture can be thought of as one gas.
– By knowing air’s pressure, volume, and temperature, the total moles of molecules in an air sample can be determined—even though they are different compounds.
Partial Pressure: Pgas
- Audio 0:38:48.952989
- The pressure of a single gas in a mixture of gases is called its partial pressure.
- The partial pressure of a gas can be calculated if – a fraction of the mixture it composes and the total pressure are known; or – the number of moles of the gas in a container of a given volume and temperature are known.
- The sum of the partial pressures of all the gases in the mixture equals the total pressure. This is known as Daltonʼs law of partial pressures.
- Ptotal = Pa + Pb + Pc + …
- Gases behave independently.
Partial Pressure: Pgas
- Audio 0:40:46.362349
- The pressure due to any individual component in a gas mixture is its partial pressure (Pn).
- The partial pressure from the ideal gas law can be determined by assuming that each gas component acts independently.
- RT Pn = nn V
Vocab
| Term | Definition |
|---|---|
| relationship between volume and moles | V = constant * n(moles) |
| standard conditions | Using these conditions 22.4 L is the volume of one mol of any gas (1 atm, 273 K (0 C), 1 mol) |
| partial pressure | the pressure of a single gas in a mixture of gases |
| Daltonʼs law of partial pressures | Ptotal = Pa + Pb + Pc + … |