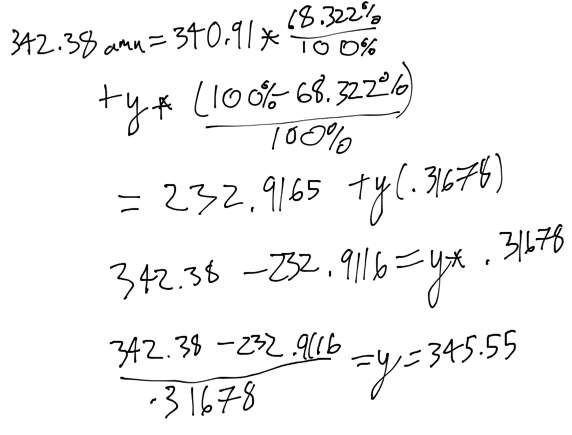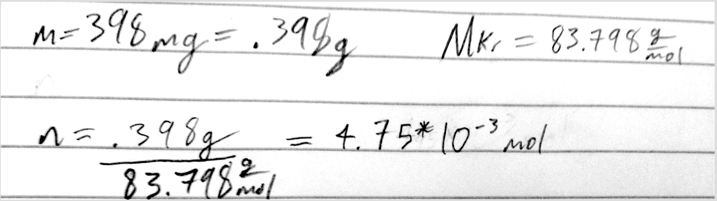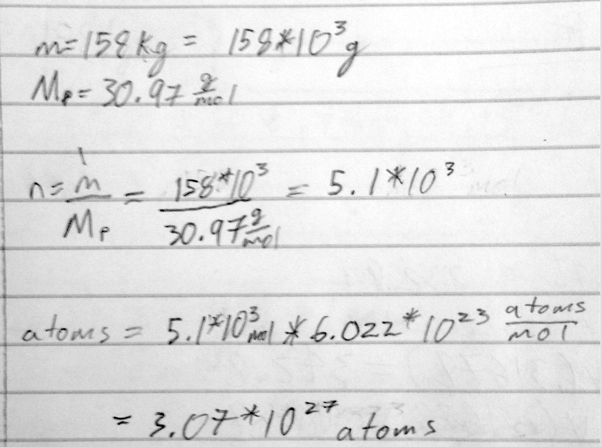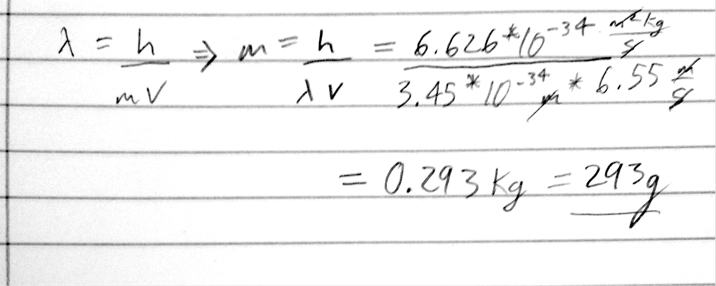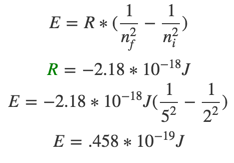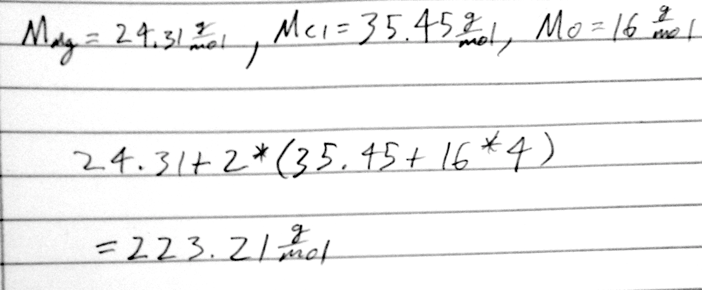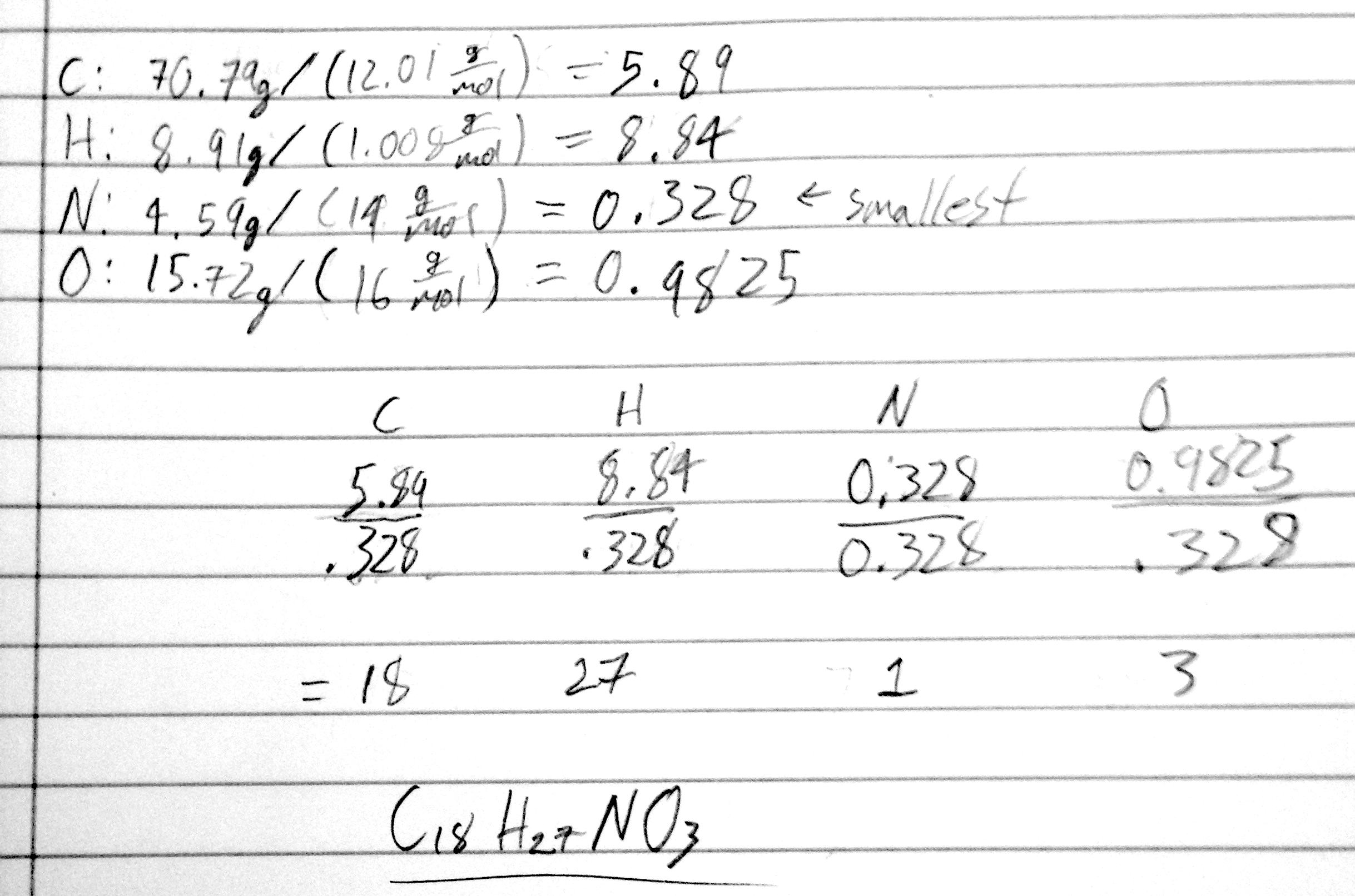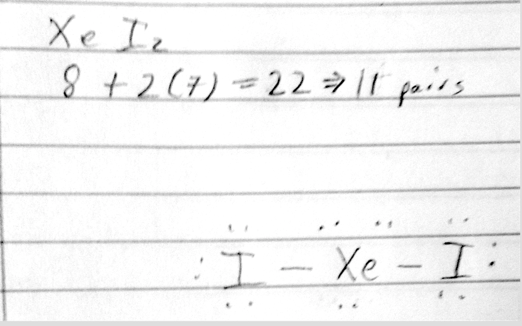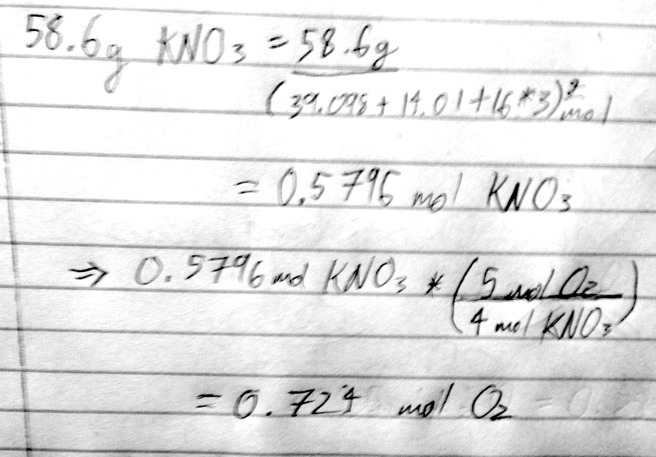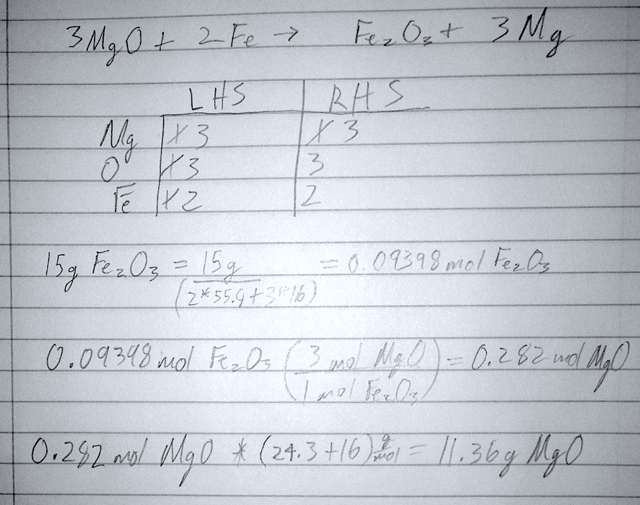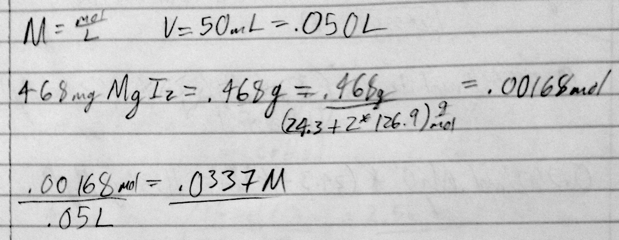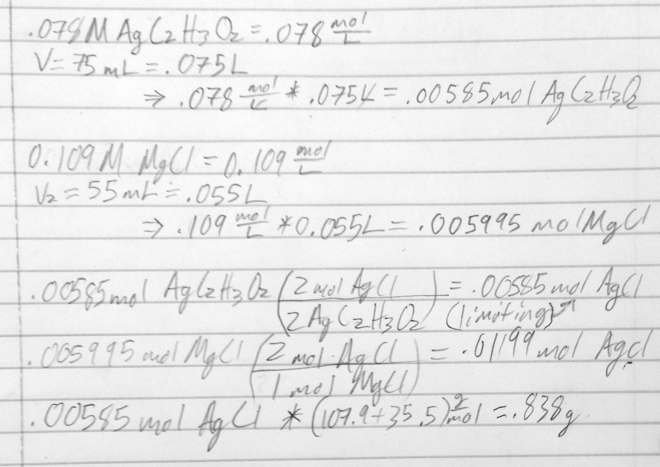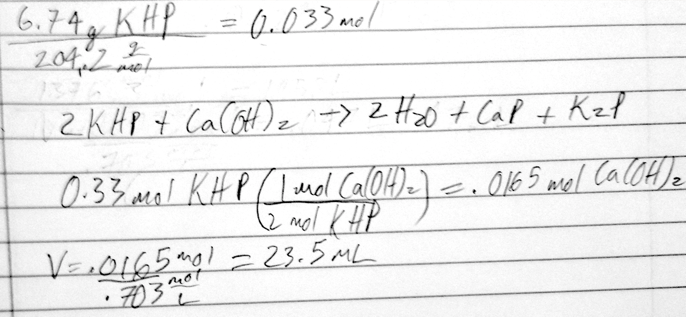Mock Final
- 1) A new compound was recently discovered and found to have an atomic weight of 342.38 amu. This element has two isotopes, the lighter of which has a mass of 340.91 amu and an abundance of 68.322%. What is the mass of the heavier isotope?
-
- A) 350.21
-
- B) 345.55
-
- C) 342.38
-
- D) 348.67
-
- E) 343.29
-
- 2) Identify the characteristics of a liquid.
- A) definite volume and definite shape
- B) definite volume and no definite shape
- C) no definite volume and definite shape
- D) no definite shape and no definite volume
- 3) What species is represented by the following information?
- p+ = 12 n° = 14 e- = 10
- A) Si4+
- B) Mg
- C) Ne
- D) Si
- E) Mg2+
- 4) How many moles of Kr are contained in 398 mg of Kr?
- A) 4.75 × 10-3 moles Kr
- B) 33.4 moles Kr
- C) 2.11 × 10-4 moles Kr
- D) 2.99 × 10-3 moles Kr
- E) 1.19 × 10-4 moles Kr
- 5) How many phosphorus atoms are contained in 158 kg of phosphorus?
- A) 3.07 × 10^27 phosphorus atoms
- B) 2.95 × 10^27 phosphorus atoms
- C) 3.25 × 10^28 phosphorus atoms
- D) 1.18 × 10^24 phosphorus atoms
- E) 8.47 × 10^24 phosphorus atoms
- 6) A student performs an experiment to determine the density of a sugar solution. She obtains the following results: 1.11 g/mL, 1.81 g/mL, 1.95 g/mL, 1.75 g/mL. If the actual value for the density of the sugar solution is 1.75 g/mL, which statement below best describes her results?
- A) Her results are precise, but not accurate.
- B) Her results are accurate, but not precise.
- C) Her results are both precise and accurate
- D) Her results are neither precise nor accurate.
- E) It isn’t possible to determine with the information given.
- 7) What answer should be reported, with the correct number of significant figures, for the following calculation? (433.621 - 333.9) × 11.900
- A) 1.19 × 10^3
- B) 1.187 × 10^3
- C) 1.1868 × 10^3
- D) 1.18680 × 10^3
- E) 1.186799 × 10^3
- 8) If an object has a density of 8.65 g/cm3, what is its density in units of kg/m3?
- A) 8.65 × 10^-3 kg/m3
- B) 8.65 × 10^-7 kg/m3
- C) 8.65 × 10^3 kg/m3
- D) 8.65 × 10^1 kg/m3
- E) 8.65 × 10^-1 kg/m3
- 9) Calculate the energy of the green light emitted, per photon, by a mercury lamp with a frequency of
- 5.49 × 10^14 Hz.
- A) 2.75 × 10^-19 J
- B) 3.64 × 10^-19 J
- C) 5.46 × 10^-19 J
- D) 1.83 × 10^-19 J
- E) 4.68 × 10^-19 J
- 10) Determine the longest wavelength of light required to remove an electron from a sample of potassium metal, if the binding energy for an electron in K is 1.76 × 10^3 kJ/mol.
- A) 147 nm
- B) 68.0 nm
- C) 113 nm
- D) 885 nm
- E) 387 nm
- 11) Determine the mass of a ball with a wavelength of 3.45 × 10^-34 m and a velocity of 6.55 m/s.
- A) 0.293 g
- B) 12.6 g
- C) 293 g
- D) 346 g
- E) 3.41 g
- 12) What are the possible orbitals for n = 3?
- A) s, p, d
- B) s, p, d, f
- C) s
- D) s, p
- 13) Determine the energy change associated with the transition from n = 2 to n = 5 in the hydrogen atom. (RH=-2.18 x 10^-18 J)
- A) -2.18 × 10^-19 J
- B) +6.54 × 10^-19 J
- C) +4.58 × 10^-19 J
- D) -1.53 × 10^-19 J
- E) +3.76 × 10^-19 J
- 14) Predict the charge that an ion formed from sulfur would have.
- A) 1-
- B) 6+
- C) 3-
- D) 4+
- E) 2-
- 15) Give the ground state electron configuration for I.
- A) [Kr]5s2 4d10 5p6
- B) [Kr]5s2 4d10 5p5
- C) [Kr]4d10 5p6
- D) [Kr]5s2 5p6
- E) [Kr]5s2 5d10 5p6
- 16) Place the following elements in order of increasing atomic radius.
- P Ba Cl
- A) Ba < P < Cl
- B) P < Cl < Ba
- C) Cl < P < Ba
- D) Cl < Ba < P
- E) Ba < Cl < P
- 17) Place the following in order of decreasing IE1.
- Cs Mg Ar
- A) Cs > Mg > Ar
- B) Mg > Ar > Cs
- C) Ar > Mg > Cs
- D) Cs > Ar > Mg
- E) Mg > Cs > Ar
- 18) Why does an electron found in a 2s orbital have a lower energy than an electron found in a 2p orbital in multielectron systems?
- A) Electrons in the 2s orbital are shielded by electrons in the 2p.
- B) There are more nodes found in the 2s orbital.
- C) Electrons in the 2s orbital can penetrate the 1s orbital and be closer to the nucleus .
- D) The larger number of electrons found in the 2p orbital leads to greater repulsion.
- E) The shape of the orbital ultimately determines the energy of the electrons
- 19) Give the structure for sodium perchlorate.
- A) NaClO
- B) NaClO2
- C) NaClO3
- D) NaClO4
- 20) Calculate the molar mass for Mg(ClO4)2.
- A) 223.21 g/mol
- B) 123.76 g/mol
- C) 119.52 g/mol
- D) 247.52 g/mol
- E) 75.76 g/mol
- 21) Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen, and 15.72% oxygen.
- A) C18H27NO3
- B) C18H27NO2
- C) C17H27NO3
- D) C17H26NO3
- 22) Combustion analysis of 63.8 mg of a C, H and O containing compound produced 145.0 mg of CO2 and 59.38 mg of H2O. What is the empirical formula for the compound?
- A) C5H2O
- B) CHO
- C) C3H6O
- D) C3H7O
- E) C6HO3
- 23) Identify the number of bonding pairs and lone pairs of electrons in water.
- A) 1 bonding pair and 1 lone pair
- B) 1 bonding pair and 2 lone pairs
- C) 2 bonding pairs and 2 lone pairs
- D) 2 bonding pairs and 1 lone pair
- E) 3 bonding pairs and 2 lone pairs
- 24) Choose the best Lewis structure for XeI2
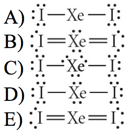
- A
- B
- C
- D
- E
- 25) Which of the following resonance structures for OCN⁻ will contribute most to the correct structure of OCN⁻?
- A) O(2 lone pairs) double bonded to C double bonded to N (2 lone pairs)
- B) O(1 lone pair) triple bonded to C single bonded to N(3 lone pairs)
- C) O(1 lone pair) double bonded to C(2 lone pairs) double bonded to N(1 lone pair)
- D) O(3 lone pairs) single bonded to C triple bonded to N(with 1 lone pair)
- E) They all contribute equally to the correct structure of OCN⁻.
- 26) Draw the best Lewis structure for the free radical, NO2. What is the formal charge on the N?
- A) 0
- B) +1
- C) -1
- D) +2
- E) -2
- 27) Determine the electron geometry (eg) and molecular geometry (mg) of CO2.
- A) eg = tetrahedral, mg = tetrahedral
- B) eg = linear, mg = trigonal planar
- C) eg = trigonal planar, mg = bent
- D) eg = linear, mg = linear
- E) eg = trigonal planar, mg = trigonal planar
- 28) Consider the molecule below. Determine the molecular geometry at each of the 3 labeled atoms.
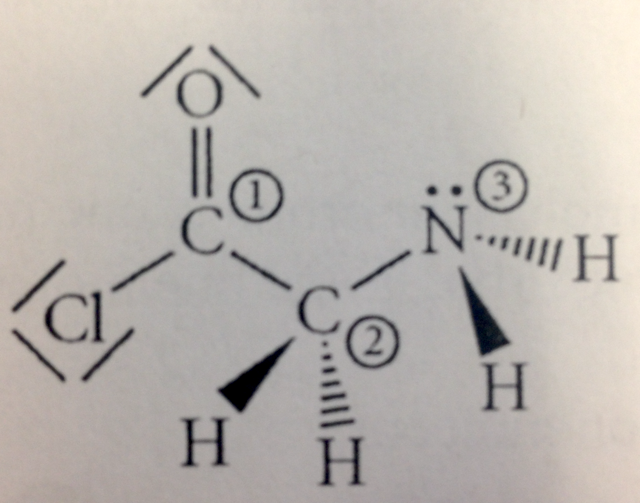
- 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
- l = tetrahedral, 2 = tetrahedral, 3 =tetrahedral
- 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
- 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
- 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal
- 29) Place the following in order of decreasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule.
- CS2 CF4 SCl2
- A) CS2 = SCl2 > CF4
- B) SCl2 > CF4 > CS2
- C) CF4 > CS2 > SCl2
- D) CS2 > CF4 > SCl2
- E) CF4 > CS2 > SCl2
- 30) Give the hybridization for the S in SO3.
- A) sp
- B) sp3
- C) sp2
- D) sp3d
- E) sp3d2
- 31) Use the molecular orbital diagram shown to determine which of the following is most stable.
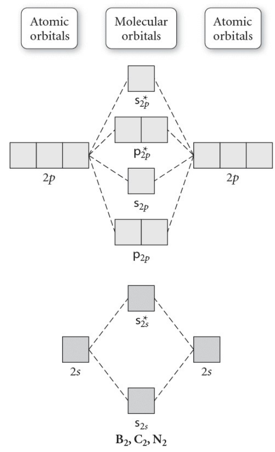
- A) C2^2⁺
- B) N2^2⁺
- C) B2
- D) C2^2⁻
- E) B2^2⁺
- 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g)
- A) 0.290 mol O2
- B) 0.580 mol O2
- C) 18.5 mol O2
- D) 0.724 mol O2
- E) 1.73 mol O2
- 33) Give the theoretical yield, in moles, of CO2 from the reaction of 4.00 moles of C8H18 with 4.00 moles of O2.
- 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
- A) 0.640 moles
- B) 64.0 moles
- C) 2.56 moles
- D) 16.0 moles
- 34) Which should give the least vigorous reaction when dropped in water?
- A) Li
- B) Na
- C) K
- D) Rb
- E) Cs
- 35) Balance the chemical equation given below, and determine the number of grams of MgO are needed to produce 15.0 g of Fe2O3.
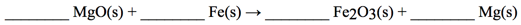
- A) 0.0877 g
- B) 1.26 g
- C) 3.78 g
- D) 11.4 g
- 36) Determine the theoretical yield of H2S (in moles) if 16 mol Al2S3 and 16 mol H2O are reacted according to the following balanced reaction. A possibly useful molar mass is Al2S3 = 150.17 g/mol.
- Al2S3(s) + 6 H2O(l) → 2 Al(OH)3(s) + 3 H2S(g)
- A) 48 mol H2S
- B) 16 mol H2S
- C) 32 mol H2S
- D) 24 mol H2S
- E) 8.0 mol H2S
- 37) Determine the molarity of a solution formed by dissolving 468 mg of MgI2 in enough water to yield 50.0 mL of solution.
- A) 0.0297 M
- B) 0.0337 M
- C) 0.0936 M
- D) 0.0107 M
- E) 0.0651 M
- 38) What mass (in g) of AgCl is formed from the reaction of 75.0 mL of a 0.078 M AgC2H3O2 solution with 55.0 mL of 0.109 M MgCl2 solution?
- 2 AgC2H3O2(aq) + MgCl2(aq) → 2 AgCl(s) + Mg(C2H3O2)2(aq)
- A) 0.838 g
- B) 1.72 g
- C) 0.859 g
- D) 2.56 g
- E) 1.70 g
- 39) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of H2SO4 and KOH are mixed
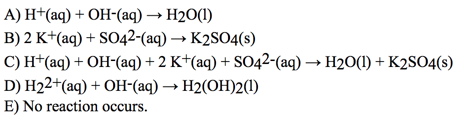
- A
- B
- C
- D
- E
- 40) Determine the oxidation state of nitrogen in NO.
- A) +5
- B) +3
- C) 0
- D) +2
- E) +4
- 41) Determine the reducing agent in the following reaction.
- 2 Li(s) + Fe(C2H3O2)2(aq) → 2 LiC2H3O2(aq) + Fe(s)
- A) O
- B) H
- C) C
- D) Fe
- E) Li
- 42) 6.74 g of the monoprotic acid KHP (MW = 204.2 g/mol) is dissolved into water. The sample is titrated with a 0.703 M solution of calcium hydroxide to the equivalence point. What volume of base was used?
- A) 8.64 mL
- B) 11.8 mL
- C) 23.5 mL
- D) 47.0 mL
- E) 93.9 mL