Week 9 - Day 3 (Ch 7 - pt 4)
Navigate using audio
Clicker 1
- Audio 0:00:26.347467
- Give the hybridization for the C in C2F2
- A) sp3d2
- B) sp3d
- C) sp3
- D) sp2
- E) sp
Period Two Homonuclear Diatomic Molecules
- Audio 0:03:34.227150
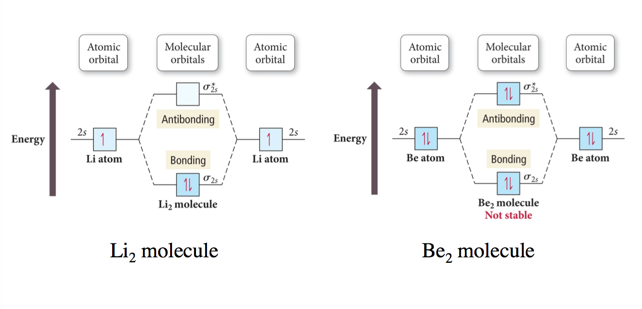
- Exactly the same as 1s
Interaction of p Orbitals
- Audio 0:06:30.224293
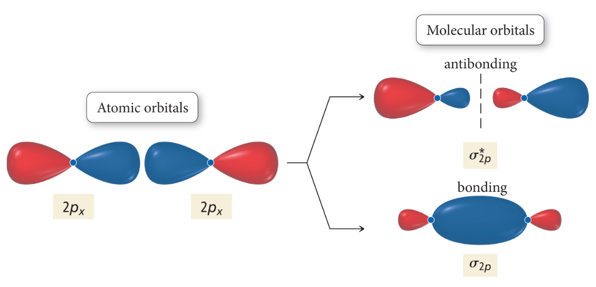
- blue bond called sigma bond because it is symmetric
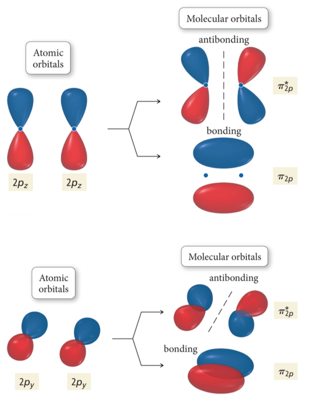
- Called 2px and 2py because of rotation
Molecular Orbital Energy Ordering
- Audio 0:10:03.432001
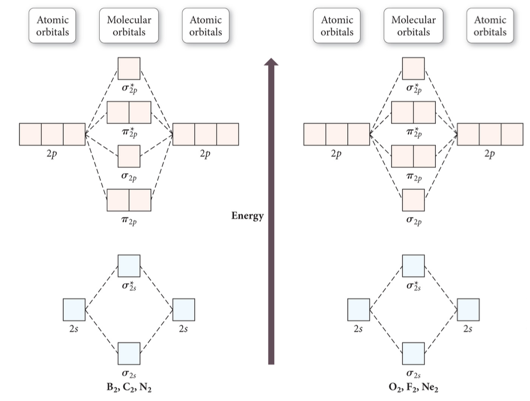
- Complete diagram for determining molecular orbital energy ordering for <= 2p orbitals
Practice Problem on Molecular Orbital Theory N2- ion. Determine the electron configuration, and whether the ion is para or diamagnetic
- Audio 0:12:43.087962
Molecular Orbital Energy Diagrams for SecondPeriod-p-Block Homonuclear Diatomic Molecules
- Audio 0:13:49.174841
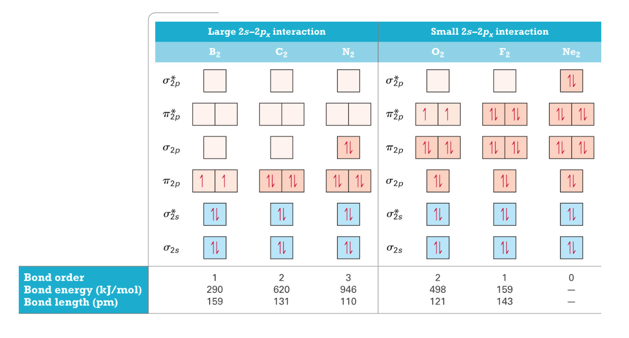
- Molecular orbital theory accurately predicts magnetism because it shows the unbonded electrons
- Also gets the bond order correct
- Audio 0:18:39.294522
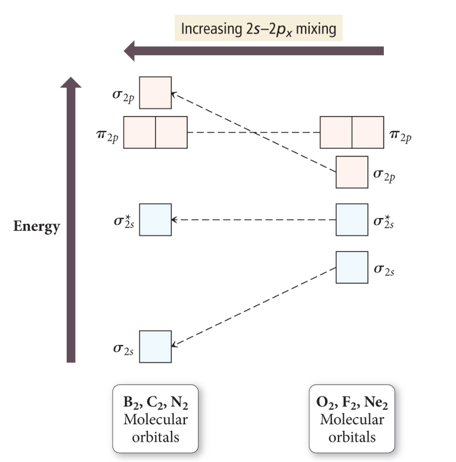
- Mixing orbitals to get optimal energy
Heteronuclear Diatomic Molecules and Ions
- Audio 0:20:09.219234
- When the combining atomic orbitals are identical and of equal energy, the contribution of each atomic orbital to the molecular orbital is equal.
- When the combining atomic orbitals are different types and energies, contributions to the MOs are different:
- The more electronegative an atom is, the lower in energy are its orbitals.
- Lower energy atomic orbitals contribute more to the bonding MOs.
- Higher energy atomic orbitals contribute more to the antibonding MOs.
- Nonbonding MOs remain localized on the atom donating its atomic orbitals.
Second-Period Heteronuclear Diatomic Molecules
- Audio 0:23:28.652003
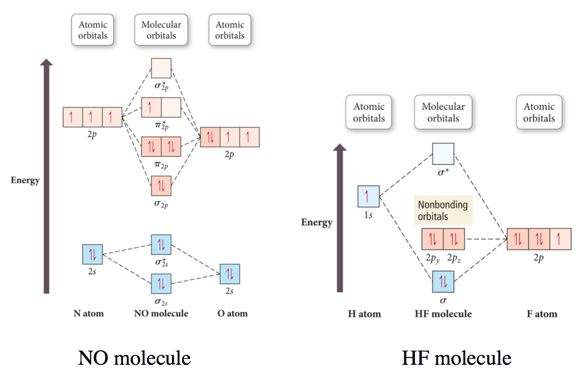
Practice Problem on Molecular Orbital Theory CN-
MO and Polyatomic Molecules
- Audio 0:26:43.466301
- When many atoms are combined together, the atomic orbitals of all the atoms are combined to make a set of molecular orbitals, which are delocalized over the entire molecule.
- Gives results that better match real molecule properties than either Lewis or valence bond theories

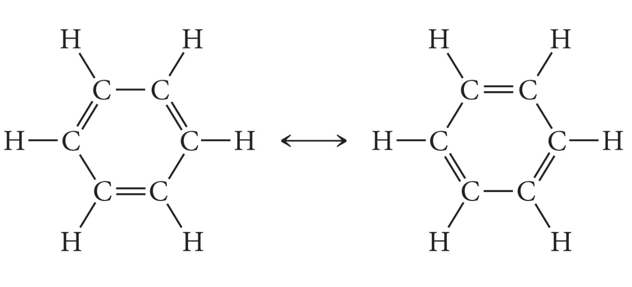
Bonding in Metals and Semiconductors
- Audio 0:28:15.434098

Clicker 2
- Audio 0:29:39.260232
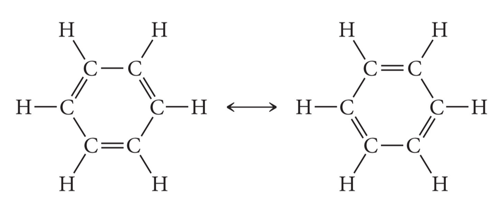
- How many p-orbitals participate in the Molecular orbitals in Benzene and how many MOs does this give?
- A) 0
- B) 2
- C) 4
- D) 6
- E) 12
Bonding in Metals and Semiconductors
- Audio 0:32:35.553208
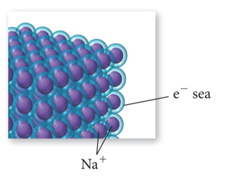
- The simplest theory of metallic bonding involves the metal atoms releasing their valence electrons to be shared as a pool by all the atoms/ ions in the metal.
- An organization of metal cation islands in a sea of electrons
- Electrons delocalized throughout the metal structure
- Bonding results from attraction of cation for the delocalized electrons.
- Audio 0:33:43.544569
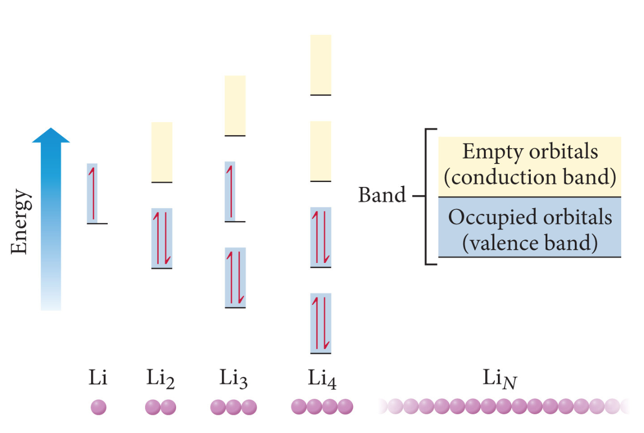
Semiconductors and Band Theory
- Audio 0:35:46.508773
- Band Theory:
- Electrons become mobile when they make a transition from the highest occupied molecular orbital into higher energy empty molecular orbitals.
- These occupied molecular orbitals are referred to as the valence band.
- The unoccupied orbitals the conduction band.
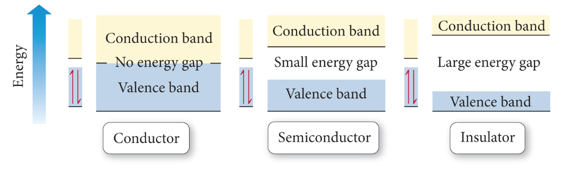
- silicon is an insulator because it has a large energy gap between the valence band to the conduction band of orbitals
- conductors have no gap
- Audio 0:37:16.124083

- Bigger the gap, the less conduction
- The funny thing is that silicon is the thing we use for conduction in transistors
- We add stuff
- The funny thing is that silicon is the thing we use for conduction in transistors
- Bigger the gap, the less conduction
- Audio 0:38:21.396460
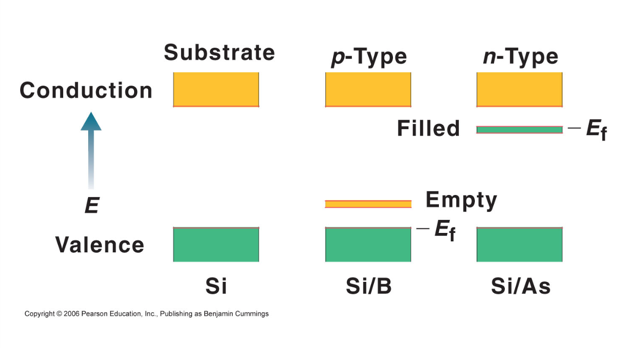
- We add a little boron and it gives an empty orbital between the valence and the conduction bonds and allows for conduction
- They can hop into the empty orbital
- We add a little boron and it gives an empty orbital between the valence and the conduction bonds and allows for conduction
End of Ch 7
- You must finish the homework
Vocab
| Term | Definition |
|---|---|
| (lower or higher?) energy atomic orbitals contribute more to the bonding MOs | lower |
| (lower or higher?) energy atomic orbitals contribute more to the antibonding MOs | higher |
| band | when many orbitals are present, their energy difference becomes relatively small and we refer to them as this |